What causes cystic fibrosis?
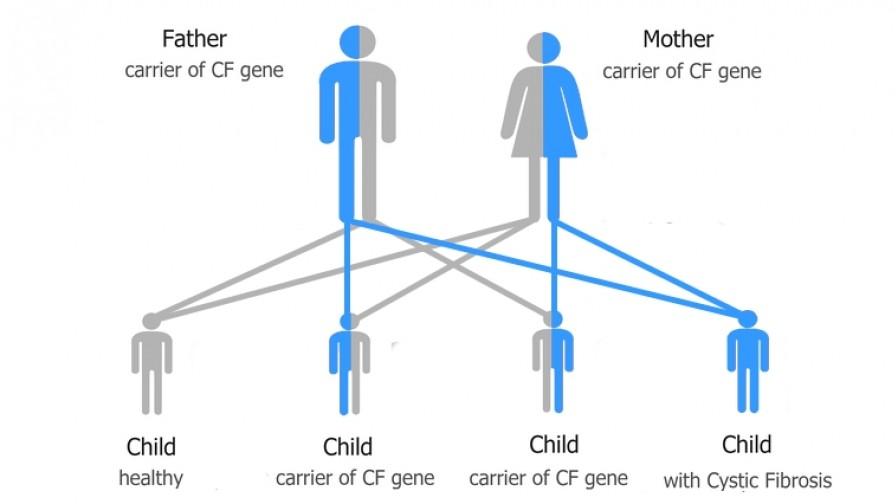
Cystic fibrosis is caused by a faulty gene that a child inherits from both of their parents - it can't be caught or developed.
One in 25 people are carriers of the cystic fibrosis gene, but for a child to be born with cystic fibrosis they have to inherit two copies of the faulty gene – one from their mother and one from their father. Parents who both carry the faulty gene have a one in four chance of having a baby with cystic fibrosis.
Current research
We are not currently funding any research projects relating to this condition
Improving treatment for lung infections
A major cause of illness and death to cystic fibrosis is persistent chest infections with a bacterium called Pseudomonas aeruginosa. Research is aiming to identify new ways to break them down.
Dr Tanmay Bharat, University of Oxford:
"Our work could open the door for enabling treatments to destroy Pseudomonas aeruginosa, helping children with CF overcome serious and potentially fatal infections.”
Previous research
Ways to support our work
Make a Donation
Your donation to Action Medical Research can help save and change children's lives.
Volunteer
Volunteers are essential to what we do. We would be very appreciative of your help across a number of important roles.
Take on a challenge
Take part in a charity event supporting Action, whatever your ambitions, sporting abilities or location.